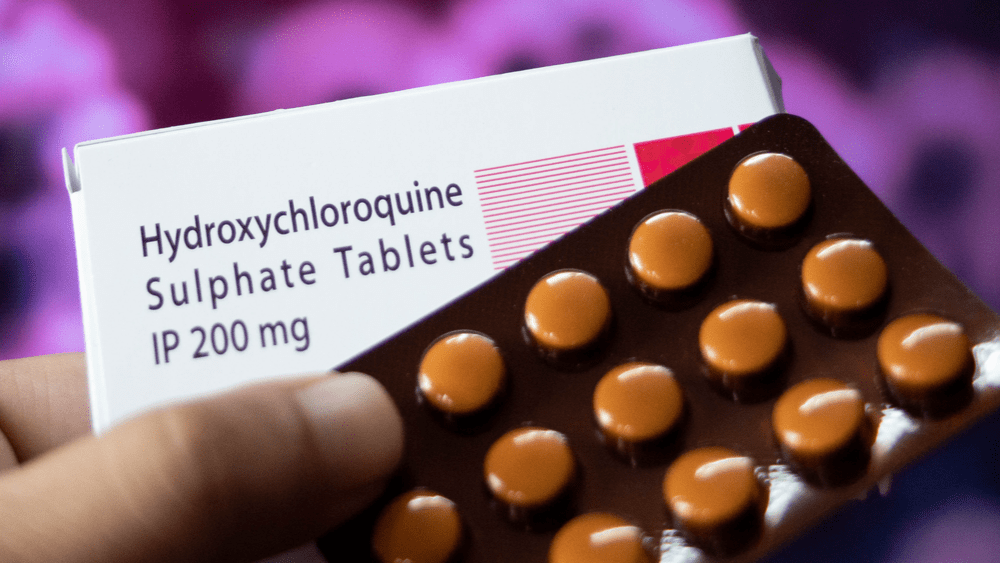
On Monday, the Food and Drug Administration (FDA) revoked the emergency use authorization for hydroxychloroquine to treat hospitalized COVID-19 patients, saying the drug carries too many risks without any apparent benefit. The EUA was first issued in March, and applied to patients hospitalized with the illness and those in clinical trials. In April, the FDA …
Continue reading “FDA Pulls Emergency Use Of Malaria Drug Hydroxychloroquine For COVID-19 Patients”